+86 178 5514 5298
+86 178 5514 5298
Leave Your Message
-
 CONTACT NUMBER
CONTACT NUMBER -
 CONTACT NUMBER
CONTACT NUMBER -
 CONTACT NUMBER
CONTACT NUMBER



In today's globalized world, the safe transportation of biological materials is more crucial than ever. The UN 3373 package is a recognized standard that ensures the safe and compliant transport of diagnostic specimens and other biological substances. Dr. Emily Jenkins, a leading expert in biocontainment and transportation logistics, emphasizes the importance of proper preparation, stating, "Adhering to UN 3373 guidelines is not just about compliance; it's about safeguarding public health and the environment."
The successful shipping of a UN 3373 package requires meticulous attention to detail and a clear understanding of the regulations involved. From packaging materials to labeling requirements, every aspect plays a pivotal role in preventing spillage and potential contamination. Inadequate preparation can lead to serious consequences, not only for the sender but also for the recipients and, ultimately, public safety.
Thus, understanding how to properly prepare a UN 3373 package is essential for any entity involved in the transport of biological materials. By following the established protocols, organizations can ensure that their shipments remain secure and considerably mitigate any risks associated with their transport. As we delve into the specifics of preparing these packages, we will explore best practices that align with UN 3373 standards to foster safe and efficient transportation.
Understanding UN 3373: Definition and Scope of Biological Substances
The United Nations (UN) 3373 designation refers specifically to the transport of biological substances that are not infectious, classified under Category B. This category includes substances such as human or animal specimens, microbiological cultures, and certain clinical samples that pose a minimal risk to public health and safety during transport. The scope of UN 3373 encompasses material that is vital for research and clinical studies but requires stringent guidelines to ensure safe handling and transportation.
According to a report from the World Health Organization, the global transport of biological materials has increased significantly over the past decade, with an estimated 22% rise in the shipping of biological substances annually. This surge emphasizes the need for proper packaging and labeling, which are essential to mitigate risks associated with leaks or contamination during transport.
Tips: When preparing a UN 3373 package, ensure that the primary container is leak-proof and placed inside a secondary container with absorbent material to contain any potential spills. Additionally, all packages must be clearly labeled with the UN 3373 marking and appropriate handling instructions to ensure safe transport.
Properly addressing compliance with international regulations is critical for organizations engaged in the shipment of biological substances. Investing in staff training and regular reviews of packaging procedures can enhance safety and reduce the likelihood of incidents during transit. Adopting best practices not only ensures compliance but also fosters trust in the handling of sensitive biological materials.
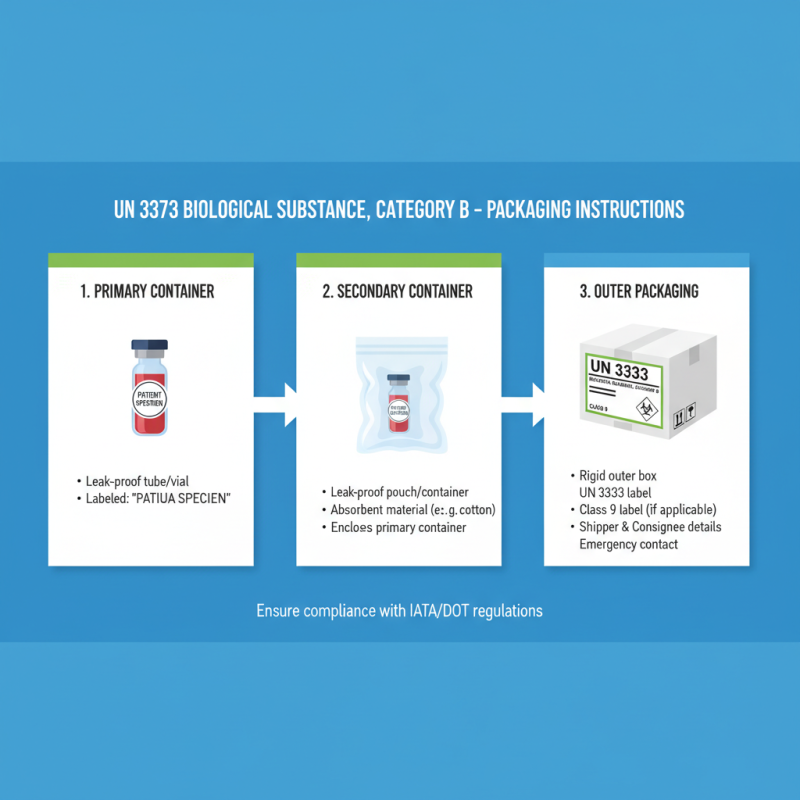
Transporting biological samples under the designation of UN 3373 requires adherence to specific regulations to ensure safety and compliance. The primary framework governing these packages falls under the guidelines established by the International Air Transport Association (IATA) and the United Nations Economic Commission for Europe (UNECE). These regulations classify UN 3373 as "Biological Substance, Category B," which is a classification meant for samples that are not infectious but still require careful handling.
To comply with these regulations, packages must meet certain performance standards, including leak-proof containers and appropriate cushioning materials to prevent breakage during transit. Packaging must also be marked with the UN 3373 label, which alerts transport personnel to the nature of the contents. Furthermore, the outer packaging must include sender and recipient information, along with any necessary handling instructions that indicate the need for special care during transport. Ensuring these elements are in place is critical to facilitating safe and lawful transportation of biological materials while minimizing risks to health and safety.
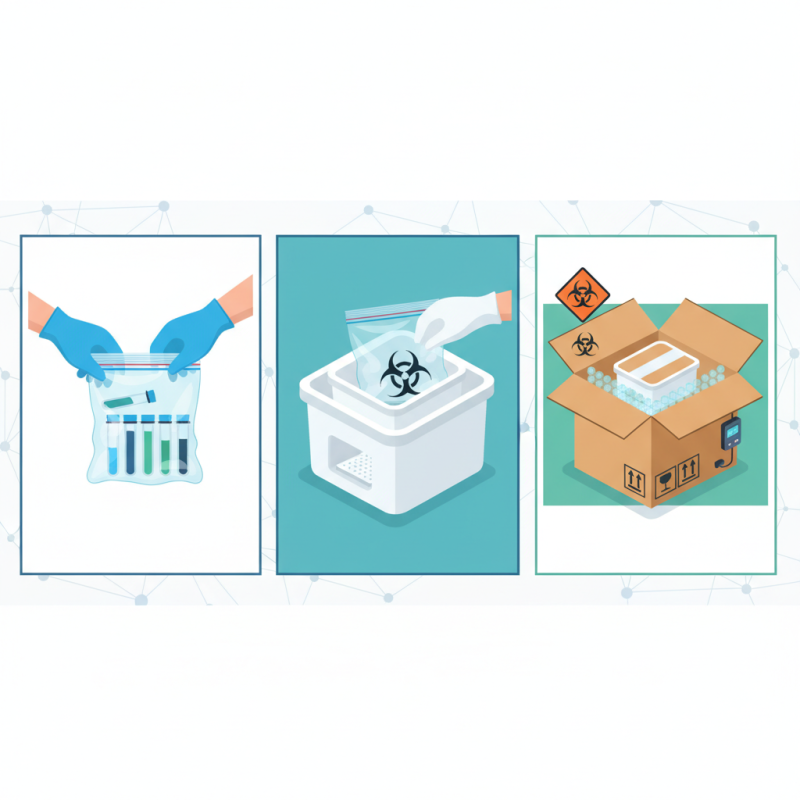
When preparing a UN 3373 package for the safe transport of biological substances, it is crucial to use the right materials to comply with packaging regulations. The primary components required include a primary receptacle, which should be leak-proof and made of durable materials. Common choices are glass or high-density polyethylene containers. This primary receptacle is then placed within a secondary container, also designed to be leak-proof, which provides additional protection against spills.
To complete the packaging, the outer packaging must be sturdy enough to withstand handling and transport. Fiberboard boxes are commonly used, but they should be strong enough to meet the weight and size requirements for the contents they carry. Additionally, absorbent material should be included within the secondary container to manage any potential leaks from the primary receptacle.
**Tips:** Always ensure that all components are properly labeled and that documentation accompanies the package. Using tamper-evident seals can add an extra layer of security and compliance. Additionally, keep in mind that packaging must also provide adequate cushioning to prevent damage during transport, so use bubble wrap or other protective materials as needed to safeguard the contents.
| Material Category | Required Material | Description | Compliance Level |
|---|---|---|---|
| Primary Container | Leaking Resistant Vials | Vials that prevent leakage during transport | Mandatory |
| Secondary Container | Rigid Packaging | Outer packaging that can withstand physical damage | Mandatory |
| Absorbent Material | Absorbent Pads | Pads that absorb potential leaks | Recommended |
| Labeling | UN3373 Labels | Labels indicating package type | Mandatory |
| Sealing Materials | Tamper-Evident Tape | Ensures package integrity | Recommended |
When preparing a UN 3373 package for safe transport, proper labeling is crucial to ensure compliance with regulations and the safety of all handling it. UN 3373 refers to diagnostic specimens, which are classified as biological substances. Begin by choosing the right packaging, which should consist of a primary container labeled clearly to indicate the type of contents, a secondary container for leakproof support, and an outer packaging that complies with the relevant specifications.
Once the containers are selected, focus on proper labeling. The package must display the UN 3373 mark—a white label with a black circle symbolizing the biological hazard. In addition, it is essential to include all necessary handling instructions such as "Biological Substance, Category B" and the appropriate warning labels. Double-check that the recipient’s and sender’s details are clearly written and easily visible on the package.
**Tips**: Always ensure that labels are securely affixed and not obscured by other tape or markings. Use waterproof materials for labels to avoid wear during transit. Consider including additional documentation, such as a declaration of the contents, inside the outer packaging to clarify any uncertainties during transport. Attention to detail in labeling not only ensures compliance but also enhances safety throughout the shipping process.

When preparing a UN 3373 package for the safe transport of biological substances, adhering to best practices is essential to ensure the integrity and safety of the samples. The United Nations defines UN 3373 as a category for biological substances, which includes clinical samples and medical waste. According to the World Health Organization (WHO), improper packaging can lead to contamination and exposure risks for both handlers and the environment, highlighting the importance of compliance with international regulations.
One crucial tip is to use appropriate primary containers, which should be leak-proof and made from materials compatible with the substances being transported. It is advisable to include absorbent material within the secondary packaging to contain any potential leaks. The International Air Transport Association (IATA) emphasizes that packaging must be robust enough to withstand the rigors of transport, and that proper labeling is key to indicating the contents and associated risks.
Additionally, always conduct a risk assessment before transport. According to the Centers for Disease Control and Prevention (CDC), understanding the biological nature of the substances, including pathogenicity and stability, allows for better planning. Ensure that the package is marked clearly with the UN 3373 label and that all accompanying documentation, such as a shipping manifest, is complete and accurate. Proper training for personnel involved in packaging and handling also plays a critical role in enhancing safety and compliance during transport.