+86 178 5514 5298
+86 178 5514 5298
Leave Your Message
-
 CONTACT NUMBER
CONTACT NUMBER -
 CONTACT NUMBER
CONTACT NUMBER -
 CONTACT NUMBER
CONTACT NUMBER



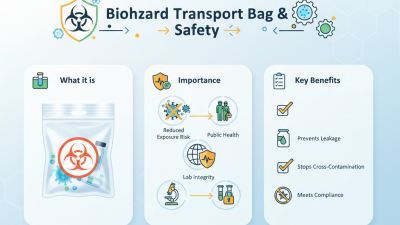
In the realm of biological research and diagnostics, the safe transport of specimens is paramount. As laboratories increasingly engage in cross-border exchanges of biological samples, understanding the proper usage of packaging protocols becomes essential. One of the key regulations governing this aspect is the UN 3373 pack, designed specifically for the safe and secure shipping of biological substances categorized as either Category B or exempt human or animal samples. Ensuring compliance with these guidelines not only facilitates the integrity of the samples but also protects public health and safety.
The UN 3373 pack provides essential protection for biological specimens during transit, mitigating risks associated with leakage or contamination. Its design includes specific components and labeling requirements that must be meticulously followed to adhere to international shipping standards. By understanding the correct usage of the UN 3373 pack, researchers can confidently transport their critical biological samples while minimizing potential hazards. This guide will explore the key aspects of using the UN 3373 pack effectively, emphasizing best practices, packaging techniques, and compliance measures that contribute to a safe shipping environment for biological materials.

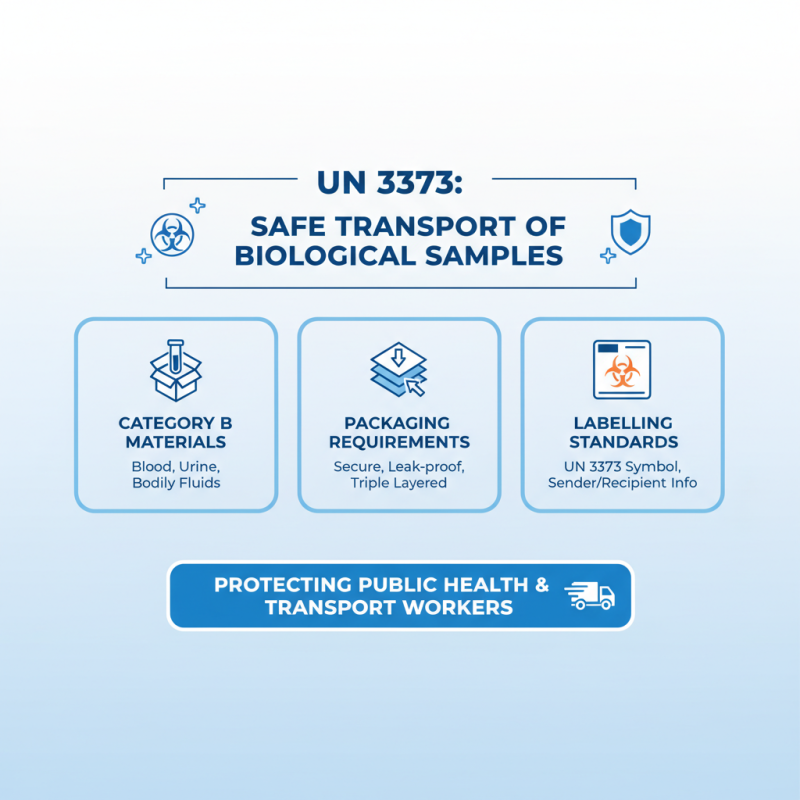
UN 3373 is a regulation set forth to ensure the safe transport of biological samples that may pose a risk to public health. It defines the requirements for packaging and labeling biological substances, known as Category B materials, which include samples such as blood, urine, and other bodily fluids. The purpose of this regulation is to minimize the risk of exposure to infectious agents and to protect transportation workers and the public during the shipping process. By adhering to UN 3373 standards, senders can ensure that their biological samples are securely contained and clearly labeled, thereby facilitating safe handling by postal and shipping services.
When preparing biological samples for shipping under UN 3373 guidelines, it is essential to use appropriate packaging that complies with the specified standards. This typically involves placing the primary container of the sample within a secondary container that is leak-proof and cushioning materials to prevent any damage during transit. Additionally, proper labeling is critical; packages must clearly indicate that they contain biological substances and include the UN 3373 mark. By understanding and implementing these guidelines, laboratories and researchers can ensure both safety and compliance, effectively transporting crucial samples without risking contamination or exposure.
When shipping biological samples, utilizing UN 3373 packaging is critical to ensure safety and compliance with regulatory standards. The essential components of UN 3373 packaging include a primary container, absorbent material, a secondary container, and an outer packaging. The primary container holds the biological sample and must be leak-proof. It should be made of a material resistant to breakage and designed to maintain the integrity of the sample during transit.
The absorbent material is crucial as it provides an additional layer of protection, absorbing any potential leaks from the primary container. The secondary container, which should also be leak-proof, houses the primary container and the absorbent material. Finally, the outer packaging must be durable and clearly labeled with the appropriate UN 3373 markings to signify that it contains biological substances.
Tips for packing biological samples include ensuring all components fit securely within the outer packaging to prevent movement during shipping. Always double-check that seal lids on the primary containers are tight to avoid any leakage. Lastly, it’s advisable to consult the latest regulations related to shipping biological materials to ensure adherence to safety standards and best practices.
This chart illustrates the essential components used in UN 3373 packaging for biological sample shipping, highlighting their importance in ensuring safe transportation.
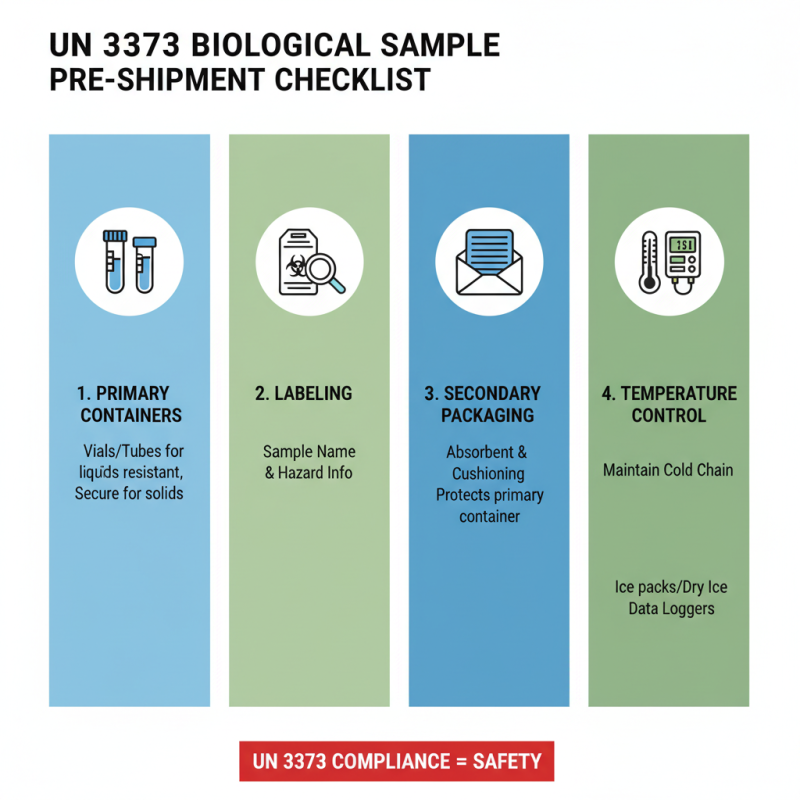
When preparing biological samples for shipping, adherence to UN 3373 guidelines is crucial to ensure safety and compliance. The first step involves selecting appropriate primary containers that are leak-proof and made of materials resistant to breakage. For liquids, use vials or tubes that can be tightly sealed; for solids, ensure they are securely contained. Each primary container should be labeled with the name of the sample and any specific hazards associated with it.
Next, the primary containers should be placed inside a secondary container that is also leak-proof. This secondary container must provide additional protection in case of breakage. It is common to use absorbent materials between the primary and secondary containers to absorb any potential leaks. After properly sealing and securing the secondary container, it must be labeled clearly as "Biological Substance Category B" with the UN 3373 symbol, ensuring that it’s easily identifiable to transport personnel. Following these steps will help ensure that samples are shipped safely and in compliance with international regulations.
When shipping biological samples under the UN 3373 regulations, proper labeling and documentation are crucial to ensuring safety and compliance. Each UN 3373 container must be labeled with a warning symbol that designates the contents as biological substances. The label typically includes the UN number (3373), a description of the material, and a cautionary statement indicating the potential hazards. It is essential to ensure the label is legible, securely attached to the package, and easily identifiable.
Documentation plays a key role in the shipping process as well. A shipping declaration may be required, providing essential details about the sample's nature, quantity, and handling instructions. This documentation must accompany the package throughout its journey and be made readily available to any authorities who may request it. Failure to complete the necessary paperwork can result in delays and complications in the shipping process.
Tips: Always double-check the labeling before dispatch to ensure that all information is accurate and complies with the regulatory requirements. Additionally, consider using a checklist that includes all necessary documentation and labeling items to streamline the shipping process and avoid oversights.
| Dimension | Description | Requirements | Example |
|---|---|---|---|
| Packaging | UN 3373 packaging should include primary and secondary containers along with outer packaging. | Must be leak-proof and appropriately marked. | Plastic tubes with caps, placed in a sturdy outer box. |
| Labeling | Labels should clearly state 'Biological Substance, Category B'. | Labels must be affixed to the outer package with a minimum size of 10 cm x 10 cm. | A symbol indicating the biological nature of the content. |
| Documentation | A declaration of contents must accompany the shipment. | A waybill and a detailed description of the contents are required. | Sender's and receiver's information along with intended contents. |
| Temperature Control | Many biological samples require temperature control during transit. | Use insulated packaging and gel packs for temperature-sensitive samples. | Gel ice packs in insulated boxes for shipping blood samples. |
| Shipping Regulations | Familiarize with the regulations specific to each carrier and destination. | Ensure compliance with IATA regulations and international laws. | Check carrier's guidelines for shipping biological samples. |
Transporting biological samples safely requires adherence to stringent guidelines outlined for UN 3373 packaging. It is essential to ensure that all samples are contained within rigid, leak-proof primary receptacles. These receptacles should be securely sealed to prevent any leakage during transit. When preparing your samples, use appropriate cushioning materials such as absorbent pads or bubble wrap to minimize movement within the package. This added layer of protection not only safeguards the integrity of the samples but also mitigates the risk of breakage.
Labeling is another critical aspect of handling UN 3373 packages safely. Each package must bear the UN 3373 mark, clearly indicating that it contains biological substances. Additionally, include relevant handling instructions to inform carriers and receivers about the nature of the contents. Proper documentation, including a declaration of contents and emergency contact information, should accompany each shipment. Following these best practices enhances compliance with safety regulations and ensures the reliable delivery of biological samples.