+86 178 5514 5298
+86 178 5514 5298 What are the general guidelines for specimen transportation?
The transportation of biological specimens is a critical aspect of medical, research, and diagnostic processes. Proper handling and transportation ensure the integrity of the specimens, accurate test results, and the safety of personnel involved. Below are the general guidelines for specimen transportation:
1. Proper Packaging
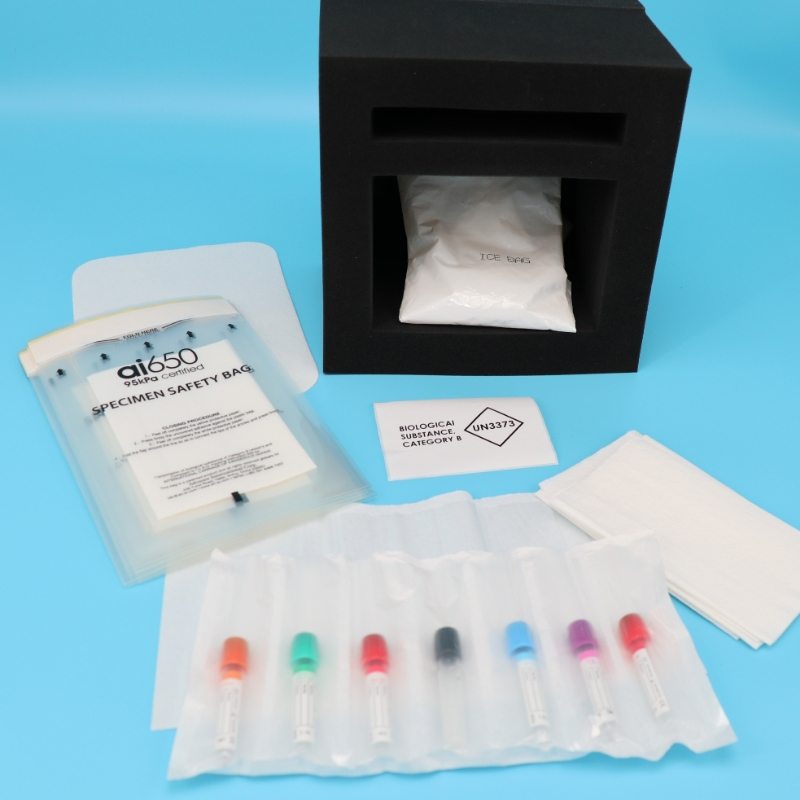
Primary Container: Specimens should be placed in a leak-proof, sterile primary container (e.g., test tubes, vials, or bottles) with a secure lid to prevent spillage or contamination.
Secondary Container: The primary container should then be placed in a secondary, durable, and leak-proof container (e.g., a plastic bag or sealed box) to provide additional protection.
Tertiary Container: For added safety, especially when transporting hazardous materials, a sturdy outer container (e.g., a cooler or insulated box) should be used. This container should be labeled appropriately and designed to withstand external pressures.
2. Temperature Control
Maintain the required temperature for the specimen during transportation. Use insulated containers with ice packs, dry ice, or temperature-controlled systems (e.g., refrigerated transport units) as needed.
Clearly label the container with temperature requirements (e.g., "Keep Refrigerated" or "Frozen").
3. Labeling and Documentation
Clearly label all specimens with patient information, specimen type, collection date, and any other relevant details.
Include a requisition form or electronic documentation with the shipment to provide context for the laboratory or receiving facility.
Use biohazard labels for infectious or hazardous materials.
4. Compliance with Regulations
Adhere to local, national, and international regulations for the transportation of biological specimens, such as those outlined by the International Air Transport Association (IATA) or the World Health Organization (WHO).
Ensure compliance with hazardous materials regulations if transporting infectious substances or dangerous goods.
5. Timely Transportation
Transport specimens as quickly as possible to minimize degradation or changes in the sample. Delays can affect the accuracy of test results.
Plan the transportation route and method (e.g., courier, postal service, or hand-delivery) to ensure timely delivery.
6. Safety Precautions
Use personal protective equipment (PPE) when handling specimens, especially if they are potentially infectious.
Train personnel on proper handling and emergency procedures in case of spills or accidents during transportation.
7. Tracking and Monitoring
Use tracking systems to monitor the location and condition of the shipment in real-time.
For temperature-sensitive specimens, use temperature monitoring devices to ensure the required conditions are maintained throughout transit.
8. Communication with Receiving Facility
Notify the receiving facility in advance about the shipment, including the expected arrival time and any special handling instructions.
Provide contact information for follow-up in case of issues or delays.
9. Special Considerations for Hazardous Materials
For infectious or hazardous specimens, follow additional safety protocols, such as using UN-certified packaging and ensuring proper documentation for regulatory compliance.
Avoid transporting hazardous materials via public transportation unless explicitly permitted.
10. Quality Control
Regularly review and update transportation protocols to ensure compliance with evolving standards and regulations.
Conduct periodic audits to identify and address potential risks in the transportation process.
By following these guidelines, healthcare providers, researchers, and laboratories can ensure the safe and effective transportation of specimens, ultimately contributing to accurate diagnoses, reliable research outcomes, and the protection of public health.